To build on the internal validation performed by VMRD, our Serum Amyloid A (SAA) test was independently evaluated by at the University of California-Davis. Blood samples were collected from horses with acute inflammation, and results were compared between sample types (blood and EDTA plasma) and to results from a reference assay. Assay linearity, repeatability, and reproducibility were also assessed. Full data will be included in a research manuscript that has been submitted for publication in a peer-reviewed journal, which will represent the first published validation of a point-of-care SAA test using whole blood. Findings of this study support the product performance claims of consistent clinical accuracy across the SAA concentration range of <20 to 3000 mg/mL with both blood and plasma, using recommended dilutions.
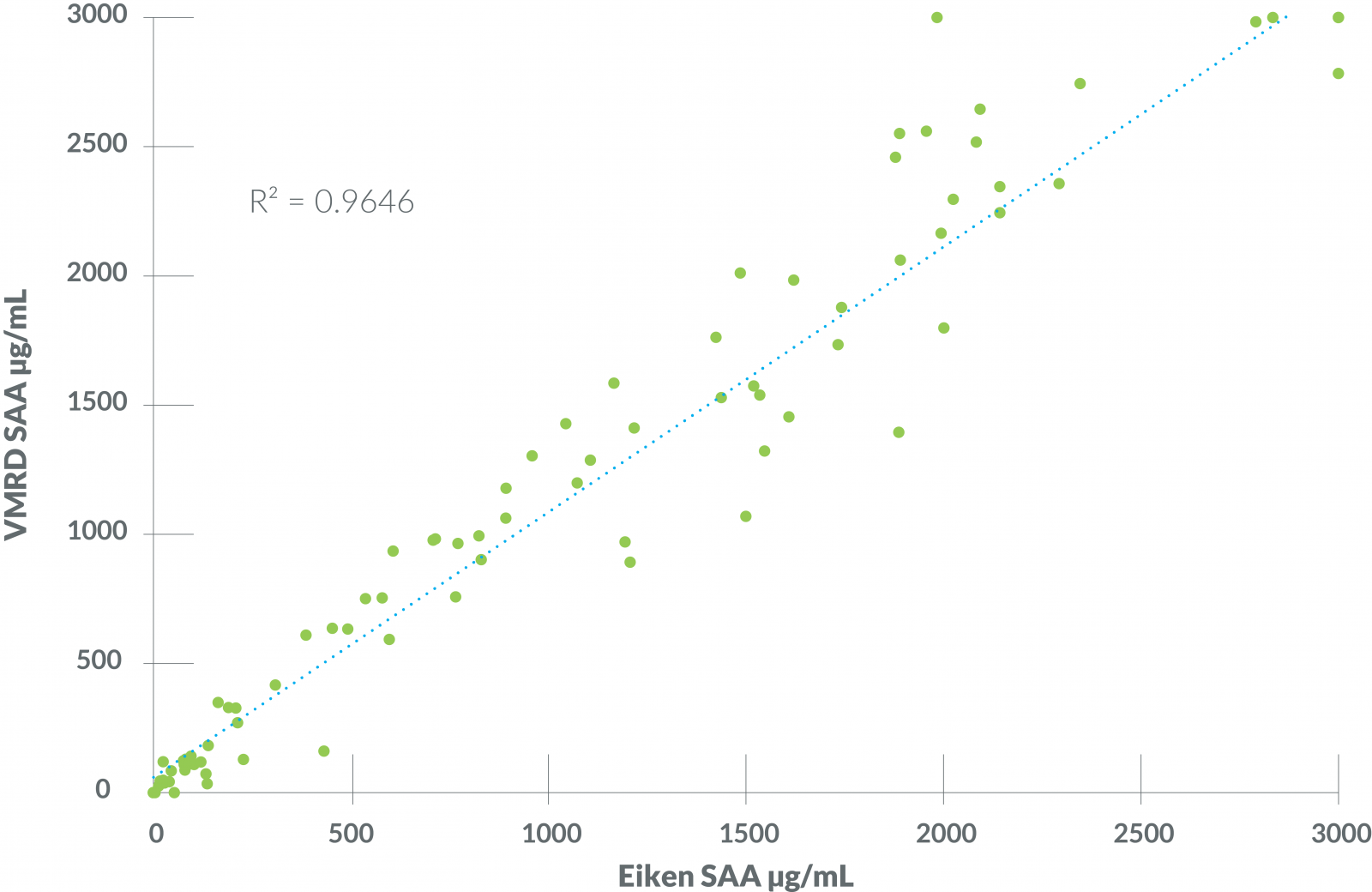
Figure 1 - VMRD SAA Test vs Eiken LZ-SAA
Results from the VMRD SAA test exhibited a strong positive correlation and agreement with the Eiken LZ-SAA assay (performed at the University of Miami Acute Phase Protein Laboratory) used as a reference.
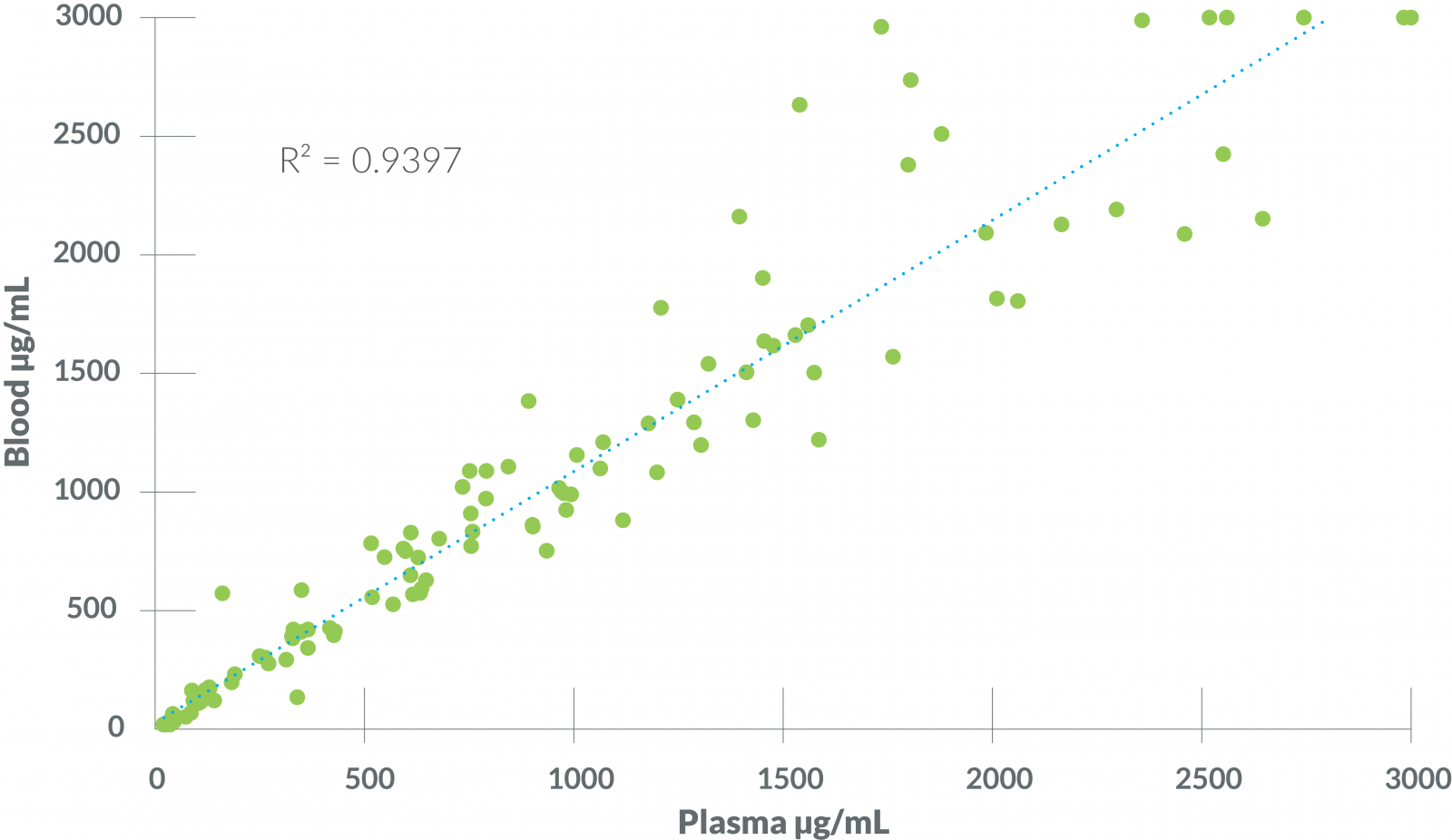
Figure 2 - Blood vs Plasma
The VMRD SAA test reliably measured SAA in both whole blood and plasma, with excellent overall agreement between paired blood and EDTA plasma samples. It is however still recommended that the same sample type be used for monitoring a clinical case over time, as exact values may vary to some degree with sample type.
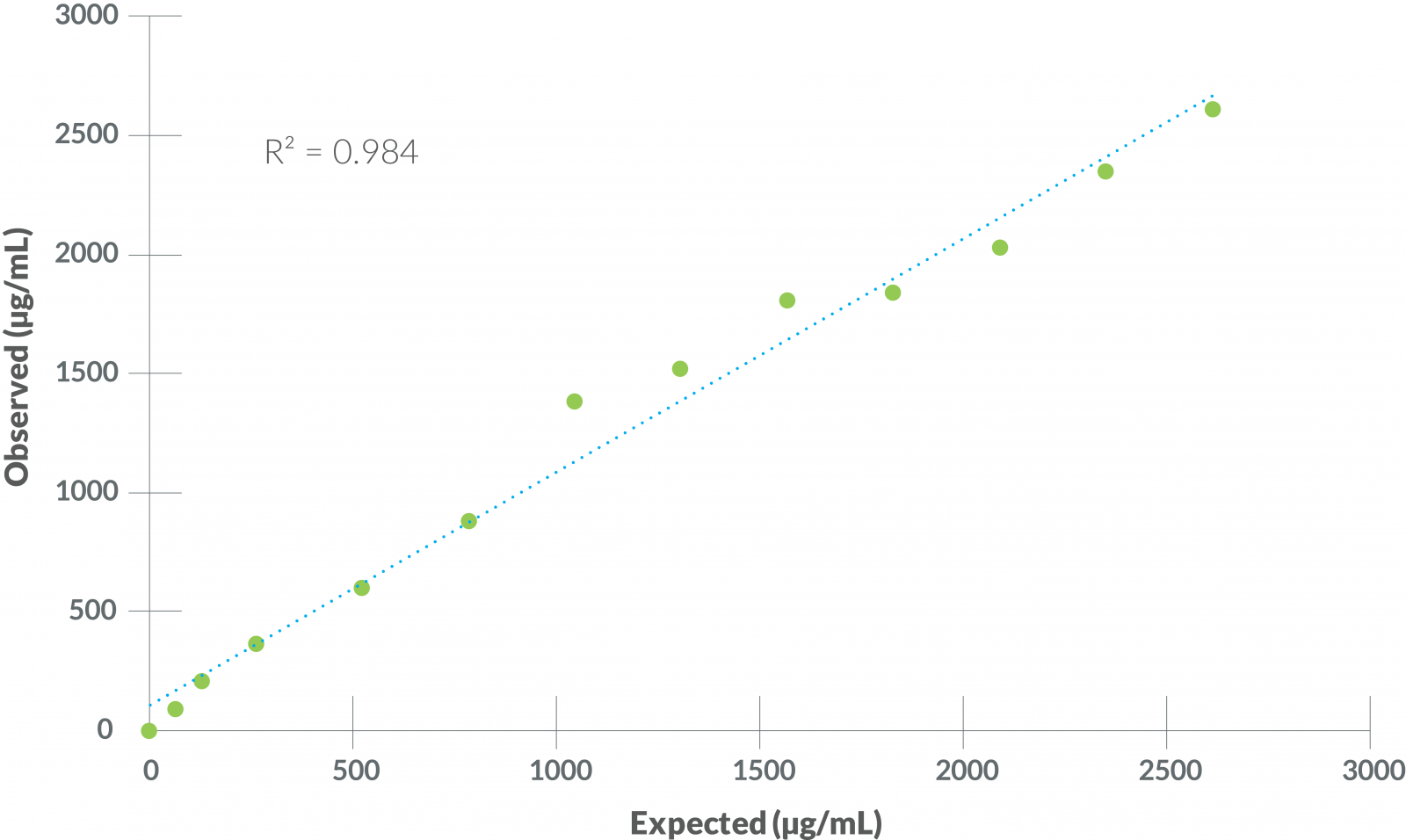
Figure 3 - Linearity
Based on serial dilution of a high SAA sample, linearity of the VMRD SAA test was solid across the entire range of dilutions, demonstrating the ability of this test to detect SAA reliably and accurately across a broad range of physiologically relevant concentrations.
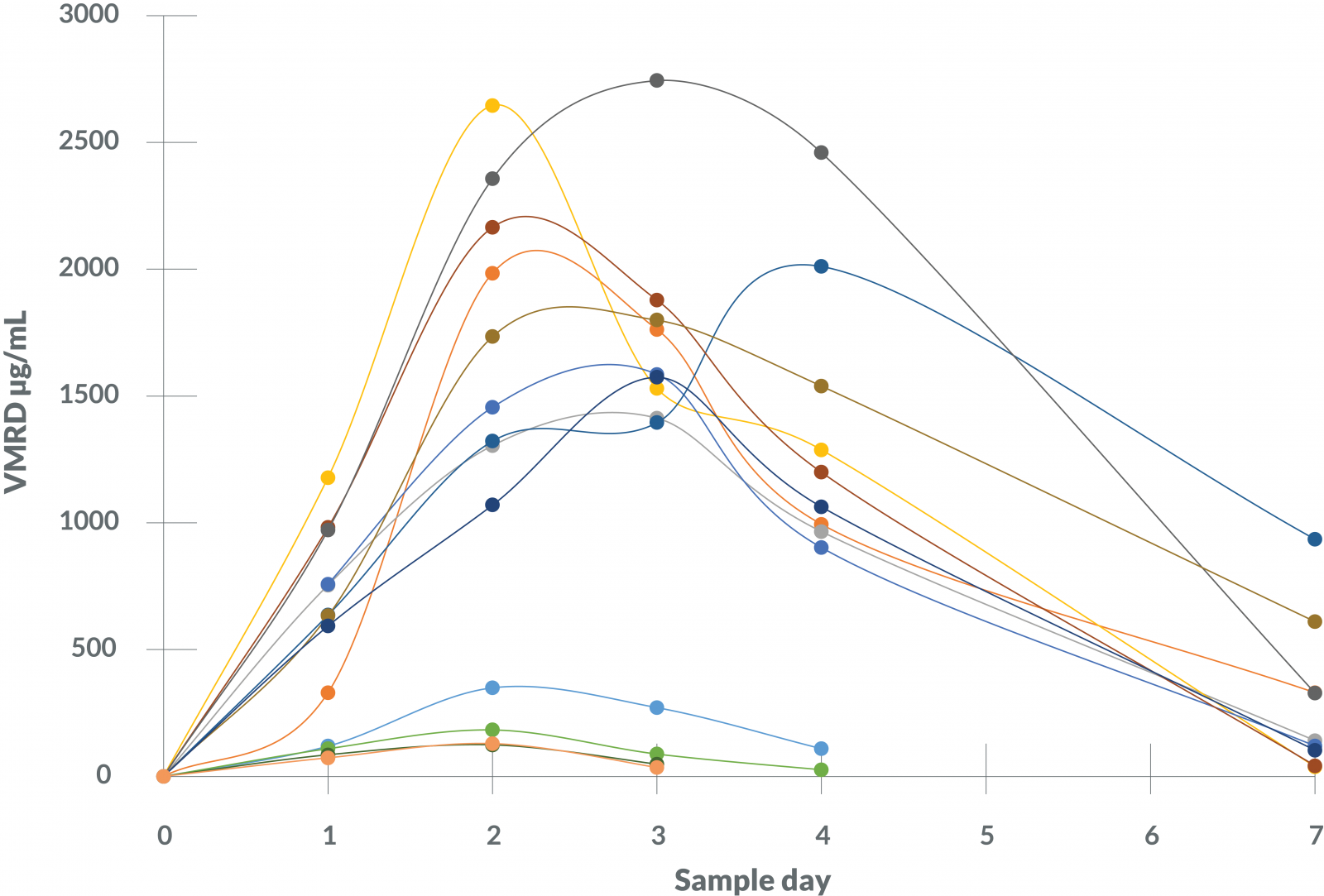
Figure 4 - SAA Levels in Individual Horses
When tracked over time in individual horses, results from the VMRD SAA test increased and decreased as would be expected following an acute inflammatory stimulus, peaking at 2-4 days and gradually decreasing over time. Comparison of VMRD and Eiken results for these horses revealed clinically similar SAA kinetics with both tests.
Evaluation of test precision revealed appropriate coefficients of variation ranging between 7.8-13.3% for repeatability (multiple tests performed on one day) and 5.7-12.0% for reproducibility (multiple tests performed on consecutive days), showing the consistency of results obtained with this test.
The SAA kit comes with the appropriate components and instructions for performing tests with either fresh or anticoagulated blood. Serum or plasma can also be used but require a different sample dilution. If interested in using plasma or serum, please contact VMRD for an appropriate protocol.
Additional studies are ongoing to further expand the capabilities of this assay – stay tuned for further updates!